Long appreciated for its benefits towards respiratory ailments, this double-blind study finds propolis effective in quickly resolving bacterial and viral upper respiratory infections, thus preventing more severe health conditions that would require pharmacological treatment. Best of all, this was documented in a randomized, controlled clinical trial, which is especially satisfying for fans of propolis.
J Phytomedicine, Volume 80, Jan 2021, 153368
Background
The most common symptoms of mild upper respiratory tract infections (URTIs) are sore throat, muffled dysphonia, and swelling and redness of the throat, which result from the inflammation process following acute bacterial or viral infection.
Hypothesis/purpose
As propolis is a natural resinous substance traditionally used to maintain oral cavity and upper respiratory tract health due to its antimicrobial and anti-inflammatory properties, the aim of this study is to evaluate the efficacy of an oral spray based on poplar-type propolis extract with a known and standardized polyphenol content, on the remission of the symptoms associated with mild uncomplicated URTIs.
Study
design
A
monocentric, randomized, double-blind, placebo-controlled clinical trial was
performed.
Methods
This study
was conducted in 122 healthy adults who had perceived mild upper respiratory
tract infections. Participants, randomly assigned to receive either propolis
oral spray (N = 58) or placebo (N = 64), underwent four visits (baseline = t0,
after 3 days = t1 and after 5 days = t2 and after a follow-up of 15 days = t3)
in an outpatient setting. Propolis oral spray total polyphenol content was 15
mg/ml. The dosage was 2–4 sprays, corresponding to 12–24 mg of polyphenols,
three times for five days. The duration of the study was 8 weeks.
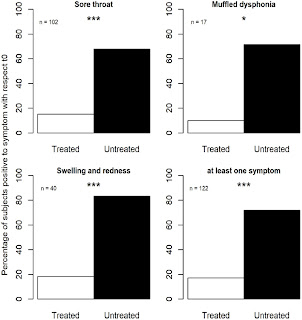
Results
After 3
days of treatment, 83% of subjects treated with propolis oral spray had
remission of symptoms, while 72% of subjects in the placebo group had at least
one remaining symptom. After five days, all subjects had recovered from all
symptoms. This means that resolution from mild uncomplicated URTIs took place
two days earlier, instead of taking place in five days as recorded in the
control group. There was no relationship between the ingestion of propolis oral
spray or placebo and adverse reactions.
Conclusion
Propolis
oral spray can be used to improve both bacterial and viral uncomplicated URTI
symptoms in a smaller number of days without the use of pharmacological
treatment, leading to a prompt symptom resolution.